The Cytotoxicity of Anti-Cancer Drug Combinations
Chemosensitivity assays for novel polychemotherapy regimens using CASY.
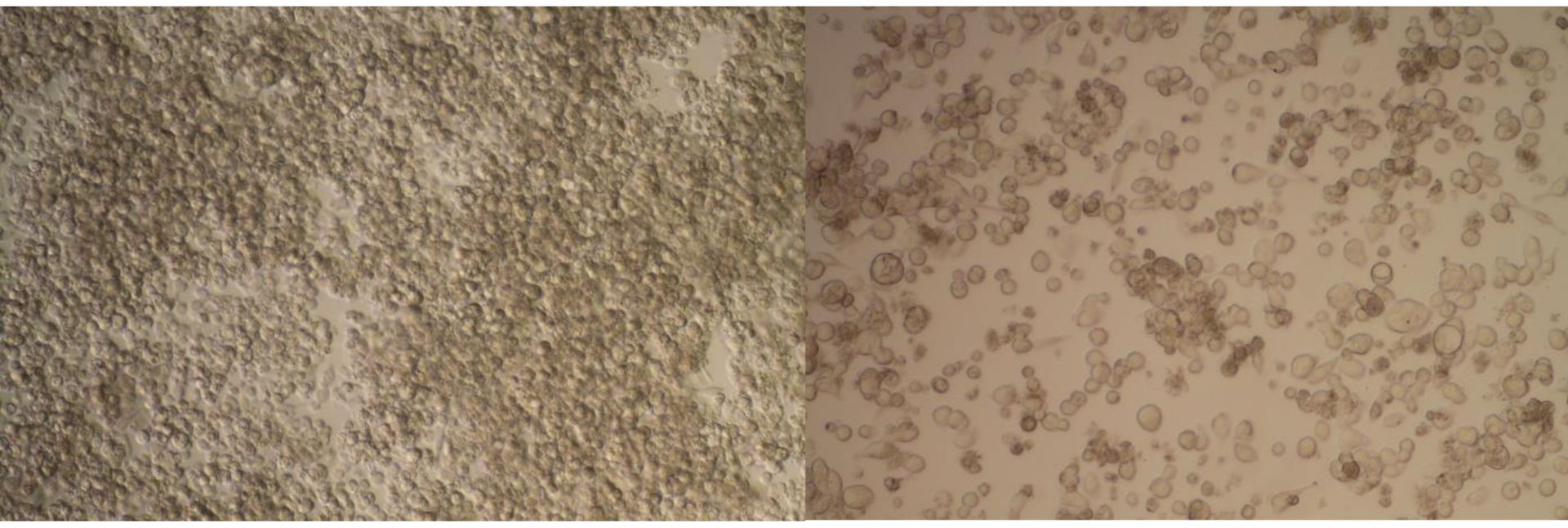
Besides CASY’s capabilities for accurate cell counting, the instrument is widely used for cell viability
and cytotoxicity measurements. Advantages are: simple and label-free cell status in just seconds
combined with excellent precision measurements and statistically relevant data.
A recent study employed CASY to evaluate the cytotoxicity of anti-cancer drug combinations in
chemotherapy. Aim was to design novel polychemotherapy regimens with wider therapeutic
windows.
Application
In a recent study by Weinreich et.al. (1), the authors examined gastric adenocarcinoma cell lines and non-malignant cell lines, treated with the standard Triple drug regimen (ECF) for gastric cancer as well as a novel duplex drug (D-D) polychemotherapy regimen. The effect of the drug combinations was evaluated using CASY growth inhibition assays: 400µL diluted cell suspensions were measured in triplicate. The cytotoxic effect of the drugs was calculated as the relative number of living cells at the start of the experiment and the cell count after incubation with various drug combinations for 5, 10 and 14 days as a percentage. The CASY evaluation window was set to include only viable cells and exclude dead cells.
Results
Evaluation of novel drug combinations. In general, CASY analysis enabled monitoring of a specific
chemosensitivity of the cell lines to the types and quantities of the combined drug regimens. A new
duplex drug (D-D) (replacing 5-FU in the standard ECF combination) has been shown to be more
effective against malignant gastric adenocarcinoma cell lines than against non-malignant cell lines.
Conclusion
CASY cell counting and cytotoxicity assays demonstrated that a new EC D-D anti-cancer drug regimen is a promising alternative to the standard ECF triple drug combination against gastric adenocarcinoma. CASY analysis revealed the differential sensitivity of gastric cancer and non- malignant cell lines to various drug combinations and allows to evaluate the anti-cancer potential of drug mixtures.

Dr. J. Weinreich, Experimentelle Onkologie, Universitätsklinikum Tübingen, Germany:
“Cytotoxicity Assays using CASY enable us to perform fast, simple and reliable
assessment of anti-cancer drug regimens and the subsequent chemosensitivity
of carcinoma cell lines. A major advantage of CASY is that debris and dead cells
can be masked in the analysis by a simple cell line specific setup and only viable
cells and their aggregates are considered. However, as a complete cell status is
recorded, also dead cells and debris data are available at any time.”

Fig. 1 CASY screenshot of MKN45 cell count. Day 0, Start of Experiment. Viability = 95.6%, 2.7*106 viable cells/mL.
LML: Viable cells/mL
VIA: %Viability
AGG: Aggregation
CL, CR: Evaluation cursors
NL, NR: Normalization cursors
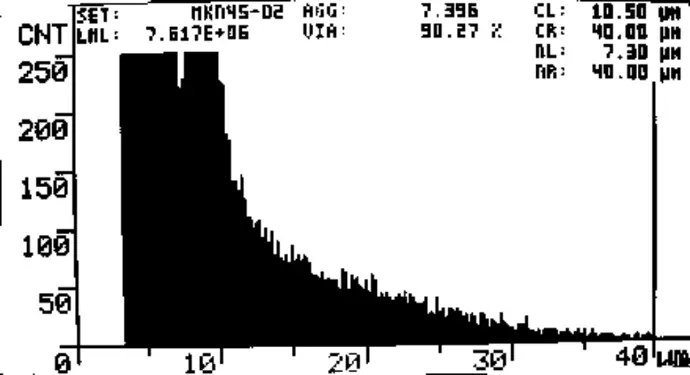
Fig. 3 CASY screenshot of MKN45 cell count. Day 7, 0.86 µmol/L D-D. Viability = 92.4%, 9.5*105 viable cells/mL
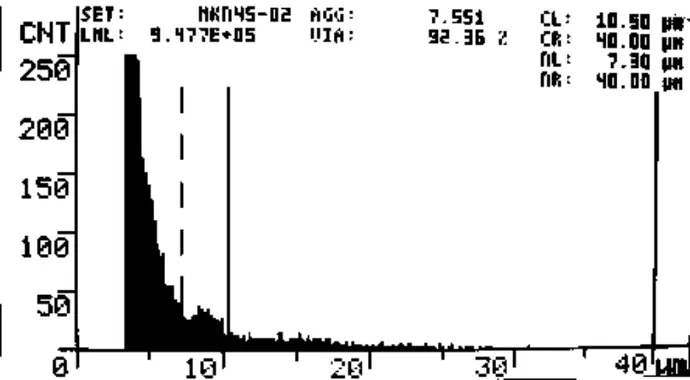
Fig. 3 CASY screenshot of MKN45 cell count. Day 7, 0.86 µmol/L D-D. Viability = 92.4%, 9.5*105 viable cells/mL

Fig. 4 Cell growth of MKN-45 cells after treatment with EC D-D.
red: E= 0,37; C=0,45; D-D=0,41 µmol/L
pink: E= 0,15; C=0,18; D-D=0,16 µmol/L
blue: E= 0,075; C=0,09; D-D=0,082 µmol/L
yellow: PBS control